Motivation of the Experiment
One of the most widely used additive manufacturing process for medal devices is Laser Based Powder Bed Fusion (LB-PBF). LB-PBF facilitates mass customisation of parts due to the design freedom and the fact that specific tooling is not needed to adjust designs. This makes it a useful tool for producing medical devices and implants, which can be tailored to the customer’s needs.
Qualifying the LB-PBF process and parts produced by LB-PBF machines for use in the highly regulated medical devices' field takes significant time and resources. There are very few published standards to guide manufacturers when it comes to part quality control, limiting the ability of the industry to be agile and to employ the MaaS models for more efficient machine usage.
Our aim is to apply the Digital Twin concept to LB-PBF processing to improve process oversite, facilitate MaaS production models and increase confidence in this production method.

Purpose of the Experiment
It has been established that parts printed on different LB-PBF machines often have physical and material variances due to the complex nature of the process. Many machines vendors have added process monitoring sensors of different types to machines to identify and correct for these variances. The analysis of sensor data in material and part development is well established on individual machines. However, to facilitate MaaS, a procedure needs to be developed to standardize the collection and processing of data from different machines in different locations, and a framework put in place to share and compare the data to aid in process validation and quality control.
3D Medlab, the end user, and IMR, the Digital Innovation hub, will be carrying out a series of experiments to identifying key indicators in the process sensor data, understanding how variation in the data impacts the quality of end use parts, and identifying what the acceptable level of variance is between 2 LB-PBF machines producing the same part. This will allow for the development of digital process models to compare the performance of different machines and improve confidence in decentralized manufacturing.
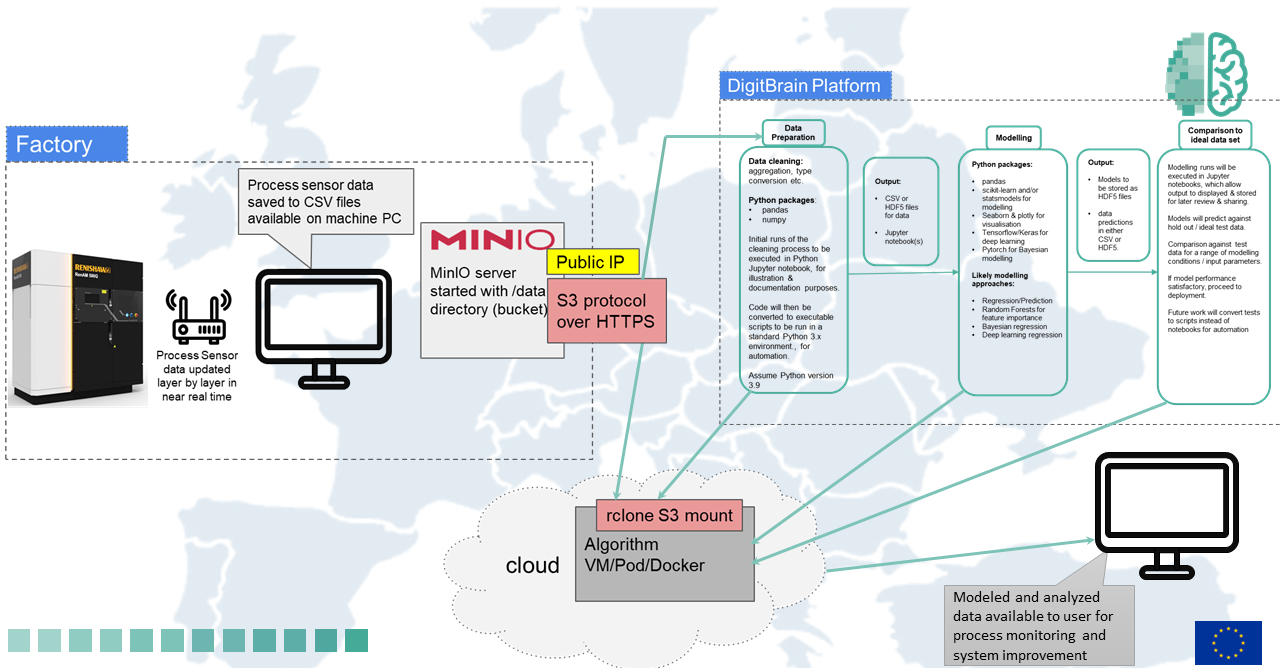
Experimental Technical Architecture of Experiment No. 4
Technical Impact
Our DIGITbrain solutions aim to improve transparency and trust in the LB-PBF process, facilitate decentralized manufacturing, and help with the development of technical standards for how the LB-PBF process is qualified and monitored. This experiment is developing a standardized method for ingesting and analysing process monitoring data so that it can be compared in a meaningful full way across multiple platforms. This will aid in the development of models for the ideal working parameters of LB-PBF machines to be used to streamline product development and increase the agility of high-performance markets like the biomedical and aeronautical sectors.
Astrid Alamercery
Collaborative Project Manager at MedLab.
"DIGITbrain project is an opportunity for us to better understand and manage additive manufacturing processes and forecast parts quality. We are happy to be part of this collaboration which enables SMEs to have access to advanced digital technologies."
Economic Impact
Reducing development time and increasing market agility helps manufacturers respond efficiently to changes in customer demands and improve market stability. 2020 has shown us the economic impact of sudden surges in demand for specialist products, and the price volatility associated with these spikes in demand. It has also shown us how dependent certain markets can be on specific supply chains, and how fragile the integrity of those supply chains can be. Improved market agility through decentralized manufacturing and MaaS production can help combat sudden changes in demand and supply, improving market efficiencies and stability.
Digital Twin Development for Validation of Multi-site Additive Manufacturing Production
Videos by the experiment:
Project Partners
3D MedLab
is the end-user in this experiment, carrying out process engineering, design, and production of medical devices in additive manufacturing
IMR
is an RTO in Ireland that will serve as the Digital Innovation Hub in this experiment.





